

Industrial production is mainly from steam reforming of natural gas, oil reforming, or coal gasification. In 1766–1781, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, the property for which it was later named: in Greek, hydrogen means "water-former". Hydrogen gas was first artificially produced in the early 16th century by the reaction of acids on metals. Because hydrogen is the only neutral atom for which the Schrödinger equation can be solved analytically, the study of its energetics and chemical bonding has played a key role in the development of quantum mechanics. The H + cation is simply a proton (symbol p) but its behavior in aqueous solutions and in ionic compounds involves screening of its electric charge by nearby polar molecules or anions. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) where it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H +.
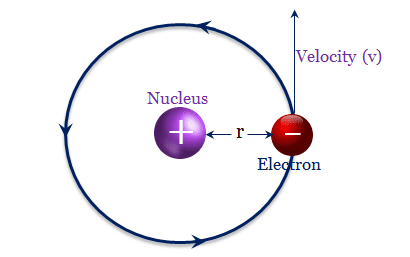
Hydrogen plays a particularly important role in acid–base reactions because these reactions usually involve the exchange of protons between soluble molecules.
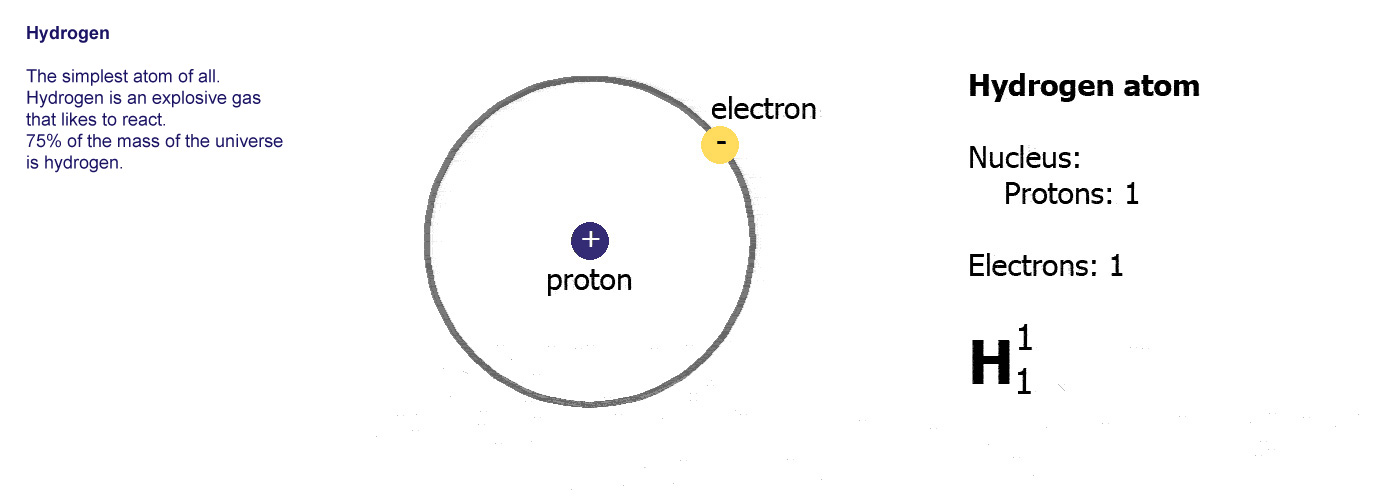
Hydrogen is nonmetallic, except at extremely high pressures, and readily forms a single covalent bond with most nonmetallic elements, forming compounds such as water and nearly all organic compounds. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 years later during the recombination epoch, when the plasma had cooled enough for electrons to remain bound to protons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. It is colorless, odorless, tasteless, non-toxic, and highly combustible. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2. Hydrogen is the chemical element with the symbol H and atomic number 1.


 0 kommentar(er)
0 kommentar(er)
